Pilot Project Program
The Center of Biomedical Research Excellence in Natural Products Neuroscience (COBRE-NPN) will fund up to five Pilot Program Projects of $50,000/year/project. The maximum duration of support will be 2 years. Applications must address one of the three areas of research (basic science, translational or entrepreneurial development) and the project scope must be within the COBRE-NPN thematic research focus area – understanding the neuropharmacologic effects of natural products.
Eligibility
Eligibility: All University of Mississippi faculty members are eligible to participate in the Pilot Project Program. Preference will be given to junior investigators who are in the early stages of their career. Those who have not yet received a major program grant (e.g. an R01) award are also encouraged to apply for the PPP. Past graduates from the COBRE-NPN program, who do not serve as mentors, may also compete for the projects but will have to present a compelling rationale to be considered for funding.
Project areas that fall within the scientific focus of the CORE-NPN include but are not limited to:
-
- Basic science research: biological evaluation of natural products as agents possessing neuropharmacologic effects.
- Translational science research: Translating basic science discoveries from animal models to human subjects.
- Commercialization (Entrepreneurial development): The advancement of research discoveries from the bench to the commercial sector.
Criteria
Criteria for evaluation of COBRE application:
-
- Initial evaluation will be done based on the cover page and specific aims submitted by all applicants and will be done by the PPP Review Committee and the EAC. The rubric for narrowing the pool is based primarily on:
- Relevance to the COBRE-NPN research focus
- Clarity of specific aims and scientific merit
- Junior Investigator status
- Proposed COBRE-NPN core utilization
- Strategic vision for leveraging PPP results into future R-type NIH funding
-
- A maximum of eight applicants will be selected. These applicants will be invited to submit a full-proposal, in R01-format, and will then participate in a Shark Tank-type meeting wherein a 10-minute presentation of the proposal is given to the PPP Review Committee who in turn provide the applicant feedback and guidance.
- Applicants will then submit a revised (A1-like) proposal to be forwarded to the EAC for final evaluation.
- The EAC will select up to five finalists pending NIGMS approval.
- Proposals will be prioritized for funding based on scientific merit, thematic research focus, career stage, collaboration teams, and the perceived likelihood of publications and competitive grant applications generated from work.
General Terms and Conditions
General Terms and Conditions of CORE-NPN COBRE Research Pilot Project Program:
-
- COBRE funds will not be released until NIGMS approves the project.
- PI or Co-I salary will not be supported. Funds may be used to support purchase of materials and supplies and for laboratory personnel salaries.
- At least 30 percent of the funds should be used for services obtained through one or both Cores (Chemistry and DMPK and/or Neuropharmacology Core).
- Pilot Project Investigators will be required to deliver a presentation every quarter on their progress.
- The investigator will be required to submit at least one abstract for presentation at a national research meeting and to submit one manuscript for peer-reviewed publication acknowledging COBRE and core support, by the end of year 1 of funding.
- Recipients will be required to submit an investigator-initiated NIH R01 grant application by the end of the second year of COBRE support. If investigator obtains external support, COBRE support will be discontinued if the COBRE supported project is significantly similar to the newly funded project.
- Investigators will be expected to present their research findings to the EAC during their annual review.
- The younger scientists, pre-R01 awardee, and assistant professors will be required to identify at least one senior faculty member to serve as their mentor. The more senior scientists at the associate or full professor rank will have the option of selecting a senior mentor to facilitate the development of their project.
- Recipients will present their work at the annual Core Research Day.
Prospective applicants with questions about eligibility, program details, application process or requirements are encouraged to contact Dr. Kristie Willett (662-915-6691; kwillett@olemiss.edu).
Current Pilot Projects

Project Title: 5HT2A agonist microdoses for pharmacotherapy of treatment-resistant depression
PI: Glenn Walker
Mentor: Dr. David Puleo
There is an urgent unmet medical need to develop therapeutic options for the ~50% of depression patients suffering from treatment-resistant depression, which is difficult to treat with existing psycho- and pharmacotherapeutic options. Classical psychedelics, such as the 5HT2A agonists lysergic acid diethylamide (LSD) and psilocybin, have re-emerged as a treatment paradigm for depression. Recent clinical trials highlight the potential effectiveness of 5HT2A agonists to improve mood and psychotherapeutic growth in treatment resistant depression patients, even in those who have failed a median of 4 previous medications in their lifetime. Accordingly, the FDA recently granted “Breakthrough Therapy” designation to psilocybin for
treatment-resistant depression. The improvements associated with 5HT2A agonist pharmacotherapy typically last for weeks to months, a marked increase relative to ketamine, but many patients find their symptoms recur and need repeat administration. ‘Microdosing’ – ingesting subperceptual doses of psychedelics every other (Q48) or every third day (Q72) to improve mood and/or creativity – represents an alternative, potentially long-term therapy for treatment-resistant depression patients. Microdosing has become prominent in the lay public, with recent scientific studies corroborating anecdotal claims that it improves mood, energy levels, social connectedness, and decreases craving for addictive substances. However, there are a gamut of practical barriers that stymie further investigation of microdosing 5HT2A agonists, including: low compliance with the complicated dosing regimen, high risk of diversion of controlled substances, and difficulty and cost administering the long-term treatment regimens in controlled settings. Here, we propose to overcome the above limitations by developing a bioresorbable microdosing implant (MDI) that enables long-term, intermittent delivery of sub-perceptual doses of 5HT2A agonists. The MDIs are fabricated by layering surface eroding polymer films composed of Cellulose Acetate Phthalate (CAP) and Pluronic F-127 (P) polymers.
These films can be layered to achieve sequential and/or intermittent drug release and the drug release rate and time between doses can be tuned by the film thickness. We will use the enabling CAPP film technology to test the hypothesis that long-term and consistent Q72 microdosing of 5HT2A agonists can be achieved by alternating 5HT2A agonist-loaded and unloaded CAPP films.
 Project Title: Microbiome Drivers of Cyanobacterial Neuroactive Natural Product Production.
Project Title: Microbiome Drivers of Cyanobacterial Neuroactive Natural Product Production.
Mentor: Vivian Hook
Cyanobacteria are an ancient clade of bacteria which are globally distributed, photosynthetic, and diverse. While they have a major role in the Earth’s carbon and nitrogen cycles, they are also of interest because genomics has shown they often bear multiple natural products biosynthetic pathways. The literature on the isolation of these cyanobacterial secondary metabolites is extensive and some of these compounds have been brought into clinical trials, but less understood is the ecological role of these compounds and how a given cyanobacterium regulates the production of multiple biosynthetic pathways. This project aims to tackle both
of these unanswered questions by using modifications of the cyanobacterial heterotrophic microbiome to change the cyanobacterial metabolome. Stressors such as temperature, salinity, and UV irradiation will drive changes in the microbiome, along with the use of antibiotics to eliminate susceptible heterotrophs from the microbiome. Cyanobacterial hosts that have had their microbiome community reduced in these experiments can then be reinoculated with individual heterotrophs isolated from the original axenic culture to see if the heterotroph can reestablish its symbiosis and elicit natural products from the host. Monitoring of
the cultures will use metagenomic sequencing, LCMS metabolomic screening, and bioassay for neuroactivity in the COBRE Neuropharmacology Core. The combined data sets will reveal not only changes in the microbiome, or the production of novel bioactive metabolites, but allow these observations to be correlated. Within the objective of driving metabolic change in the host through the microbiome community, the LCMS screening will show those changes in the metabolome and the metagenomic sequencing will reveal what heterotrophs might be responsible for those changes. When the metabolome of a cyanobacterium changes under different growth conditions as observed by LCMS comparing extracts from those two conditions by bioassay will be used to assign the difference in chemical composition to differences in the bioassay result. These assignments will then be validated by isolating and testing the natural product.
 Project Title: Plant Essential Oil Components that Interact with the Cannabinoid Receptor CB2
Project Title: Plant Essential Oil Components that Interact with the Cannabinoid Receptor CB2
PI: Dr. Xing-Cong Li
Mentor: Dr. Samir Ross
The human endocannabinoid system is a biological signaling system in the brain that regulates numerous physiological and cognitive processes. It is a promising therapeutic target for many neurological diseases. In particular, cannabinoid receptor type 2 (CB2) that is highly expressed in peripheral immune tissues and to a lesser extent in neurons and microglial cells plays a critical role in pain, anti-inflammation, and neuroprotection. We have identified seven plant-derived essential oils (EOs) possessing potent CB2 binding affinity, which may explain the medicinal properties of these EOs in aromatherapy to relieve neurological and psychiatric disorders. A preliminary chemical analysis showed that these seven EOs contain new chemotypes of cannabinoid CB2 ligands. The proposed research seeks to identify these unique phytocannabinoids that may be utilized as lead compounds for the development of a new treatment to manage depression, a major psychiatric condition affecting hundreds of millions in modern society. A novel natural products drug discovery approach will be employed to achieve this goal in an efficient way. Specifically, we will perform gas chromatography-mass spectrometry analysis to quickly determine candidate compound structures in the seven EOs. We will then conduct a virtual screening to rapidly prioritize possible active compounds. These
compounds will be acquired from commercial sources or by isolation and chemical synthesis to test their CB2 affinity, in vitro pharmacological activities, and in vivo anti-depressant efficacy in a mouse model. This approach is innovative because it reduces the conventional natural products drug discovery requirement for tremendous efforts in dereplication, isolation, and structural elucidation. In addition, a lead compound will be selected for structural optimization. A structure-based approach will be utilized to design and synthesize novel compounds with improved CB2 affinity and druggable properties. Successful completion of the project will result in the identification of structurally diverse naturally occurring cannabinoids, synthetic cannabinoids with improved CB2 affinity, and one or two lead compounds with confirmed in vivo anti-depressant efficacy, which will lay a solid foundation for future drug development.
 Project Title: Could vitamin E acetate oil be responsible for the marijuana vaping illnesses?
Project Title: Could vitamin E acetate oil be responsible for the marijuana vaping illnesses?
PI: Mona H. Haron
Mentors: Dr. Samir Ross and Dr. Shabana Khan
The outbreak of lung injury associated with the use of E-cigarette or vaping products (EVALI) on the summer of 2019 brought to the spot light the possible dangerous of smoking/inhaling cannabis/marijuana and tetrahydrocannabinol (THC). As of February 18, 2020, a total of 2,807 hospitalized EVALI cases or deaths have been reported to CDC from all 50 states, the District of Columbia, and two U.S. territories (Puerto Rico and U.S. Virgin Islands). Sixty-eight deaths have been confirmed in 29 states and the District of Columbia. Vitamin E oil in marijuana and tetrahydrocannabinol (THC) vape is currently a key focus of investigators looking into vape-associated lung injures. Human use of THC is usually via inhalation of cannabis combustion products (smoking) for its psycho-activity. Although cannabis is illegal at the federal level in the United States, products e.g. marijuana vape-pens are readily available for purchase on the Internet, at head shops, and dispensaries in states where medical or recreation cannabis is legal. Vaporizable hash oil and THC waxes were reported to deliver higher THC concentrations than combustible cannabis by 4–30 times; causing stronger exposure experience to THC. Several cases of severe illnesses related to vaping, in patients ranging in age from 15 to 46 were reported where in each case; the patient had used “at least one”
cannabis-related product. In clinician’s reports many of the illnesses was linked to people using marijuana vaping. Vitamin E oil was found as diluent in most of the marijuana but not the nicotine-based vaping products that were tested in relation to vaping illness outbreak. These findings made vitamin E acetate an agent of interest in causing those illnesses. Vitamin E is a dietary supplement that is used in some skin products with minimal side effects but its health effects are not clear when smoked alone or with THC. The rational of this study is, since in some of the cases the duration between the patients starting vaping and the onset of symptoms is very short, it is possible that the illnesses are happening due to a local (direct) effect on the lungs as well as a central (indirect) effect via cannabinoid receptors (CB) in the brain. Vitamin
E oil presence with THC in some vaping products could be causing very high levels of THC exposure by the brain via enhancing it is solubility and increasing its bioavailability. This could be predisposing and contributing to the onset of the reported illnesses. The objective of this study is determining the brain, heart and lung bioavailability and CB1 and CB2 binding affinities of THC, vitamin E acetate and their combination in concentrations similar to that present in marijuana vaping products.
 Project Title: Discovery of potential disruptors of CB1R:5HT2AR heteromer to mitigate the THC-induced cognitive impairment
Project Title: Discovery of potential disruptors of CB1R:5HT2AR heteromer to mitigate the THC-induced cognitive impairment
PI: Amar G. Chittiboyina, Ph.D., FRSc
Key members: Drs. Pankaj Pandey, Osman Galal
Mentor: Professor Robert J. Doerksen
Lately, cannabis (Cannabis sativa) consumption for medical and recreational purposes has been hotly debated, and the passage of the 2018 Farm Bill on industrial hemp (C. sativa with <0.3% THC) allowed consumers to readily access such materials for the management of various medical symptoms including neuropathic pain. Cannabis contains more than 600 phytoconstituents; among them, ~120 constituents are recognized as cannabinoids and another 120+ compounds as terpenes. Many of these constituents are recognized to have deleterious and(or) potential therapeutic effects, which are often mediated through the endogenous cannabinoid system. THC, the main psychoactive compound in cannabis, acts as a cannabinoid receptor type 1 (CB1) agonist and is associated with potential therapeutic applications, including analgesic, anxiolytic-like, and neuroprotective attributes. However, in addition to THC’s abuse potential, THC also induces numerous undesirable behavioral effects, including memory impairment and anxiogenic effects. Serotonin 2A receptors (5HT2A) are reported to modulate THC’s effects on acute amnesia, anxiety, social interaction and hence limited cannabis use and effectiveness as an analgesic. However, data for mice lacking 5HT2A suggest that the analgesic and amnesic effects of THC are independent of each other; that is, THC-induced amnesia is absent in the mutant mice, yet THC still promotes analgesia in these animals. This provides a welcome opportunity to target a receptor population that produces and(or) regulates the deleterious effects of THC. Indeed, recent data suggest that pharmacological targeting of the unique CB1:5HT2A heteromer may be exploited as a potential target to overcome THC-induced side effects.
We propose that some of the minor cannabinoids and terpenes, including CBD and limonene, can bind and regulate both CB1 and 5HT2A, which would allow us to disrupt the formation of the CB1:5HT2A heteromer, and afford the potential to dissociate the deleterious effects induced by THC from its beneficial antinociceptive properties. To this end, we will implement structure-based drug design techniques for the construction and validation of the CB1:5HT2A heteromer complex; identification of “hot-spot residues” at the interface of the heteromer, and perform protein-target-based virtual screening of public databases and all phytoconstituents of cannabis to discover potential disruptors/inhibitors of the CB1:5HT2A heteromer.
Based on these structural studies, the role of identified potential disruptors, including naturally occurring components of cannabis (cannabinoids, terpenes, and others) for their inhibitory effects on heteromer, will be explored and further validated with behavioral studies on mice to separate antinociceptive effects from cognitive effects induced by THC.
Past Pilot Projects
 Dr. Alberto Jose Del Arco Gonzalez
Dr. Alberto Jose Del Arco Gonzalez
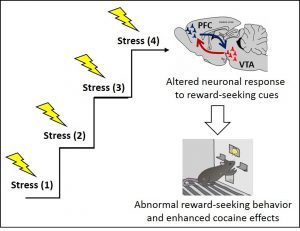
From Stress to Drug Addiction: The Neuronal Networks that Underlie Vulnerability to Drug Abuse
The repeated exposure to stressful experiences makes subjects more vulnerable to develop drug abuse. Vulnerability to drug abuse is associated with the abnormal function of the brain reward system which promotes drug-seeking behavior and enhances the reinforcing effects of drugs (drug sensitization). However, how stress alters the reward system to transform a healthy brain into a vulnerable brain is poorly understood. In preliminary experiments I find that exposing rats to repetitive intermittent social defeat stress (RISD) alters reward-seeking behavior. In line with these findings, previous studies have shown that RISD produces cocaine sensitization and promotes cocaine self-administration. Yet the neuronal networks that explain these behaviors are still unclear. The main goal of this project is to identify the neuronal networks in the brain that lead to abnormal reward-seeking behavior and enhance the effects of drugs during repetitive exposure to stress. Specifically, I will record in vivo neuronal activity in the prefrontal cortex (PFC) and the ventral tegmental area (VTA) since both areas of the brain process rewarding stimuli and regulate the response to stress.
The central hypothesis of this proposal is that the repetitive exposure to social stress changes how neurons respond to (i.e. encode) reward-seeking cues in the PFC and the VTA. These neuronal changes alter reward-seeking behavior and enhance the reinforcing effects of drugs, making subjects more vulnerable to develop drug addiction. To test this hypothesis:
Specific Aim 1 will determine the neuronal response to reward-seeking cues during social stress. Specific Aim 2 will determine whether cocaine enhances the neuronal response to reward-seeking cues after social stress.
Importantly, this project will take advantage of the Neuropharmacology CORE-NPN to carry out a multilevel experimental design in which the neuronal activity in the brain after stress is associated with changes in other behavioral and molecular parameters such as motor activity (open field test), rewarding effects (conditioned place preference test), anxiety behavior (plus maze test) and the activity of stress hormones (I.e. corticotropin-releasing factor, corticosterone) in the brain (immunohistochemistry and western blot analysis). Furthermore, the results of this research will help to develop more competitive grant applications to secure NIH R01 funding and promote collaborations between our campus and the Medical School.
Mentor: Dr. Samir Ross & Dr. Larry Walker
Novel and Selective CB2 Agonist/Inverse Agonist for Targeting Neurodenerative Disorders
The cannabinoid receptor subtype 2 (CB2) is emerging as a potential target for the development of new therapeutic approaches in several neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). CB2 is expressed by peripheral immune cells and mediates the immunosuppressive effects of cannabinoids and endocannabinoids. Recently, we have developed a new class of selective CB2 ligands as lead compounds for neuroinflammatory and neurodegenerative disorders. Pyrrolo[2,1-c][1,4] benzodiazepines (PBDs) are a class of natural products obtained from various actinomycetes which have both anti-tumor and antibiotic activities. Closer inspection of the newly designed PBD tricyclic skeleton in analogy to the natural CB ligands backbone such as THC and CBN, has sparked an innovative interest in this under explored class of the compounds as a potential source of CB2 selective ligands. We hypothesize that PBD analogs should have the proper configuration that gives the molecules a right-handed twist which will offer the appropriate three-dimensional conformation to fit snugly within the active site of CB receptors, enabling them to interfere with the endocannabinoid signaling system. Interestingly, computational studies and theoretical binding affinities of six selected (S,E)-11-[2-(arylmethylene)hydrazono]-PBD analogs, have revealed the presence of a selective tight binding attraction towards CB1 and CB2 which have been confirmed via testing. Intriguingly, one of the synthesized compounds showed a selective binding affinity towards CB2 (Ki = 8.4 nM) when compared to CB1 (Ki =65.4 nM). Our finding suggested that introduction of a halogen on the aromatic ring promotes the selectivity of CB2-ligand interaction while introducing a polar side-chain on the aromatic ring escalates the selectivity towards CB1 receptor. This in turn motivates us to move forward with optimizing our scheme for amplifying the selectivity through the generation of twenty four (S,E)-11-[2-(arylmethylene)hydrazono]-PBD analogs supported by computer-based docking studies and the drug-likeness properties via computational calculation of ADMET and physicochemical properties. The generated ligands will be evaluated for their CB receptor binding and GTP–[35S] assays as well as neuronal viability assessment in CORE-NPN. The most potent analogs will be tested in Alzheimer’s mouse model, J20 (PDGF-APPSw,Ind). The outcome of these studies has the potential to lead to potent therapeutic agents for neurodegenerative diseases and will open the window for future exploratory funding opportunities.
Development of Novel Salvinorin Compounds with 6, 5-fused Rings at C-2
The opioid system plays a crucial role in the modulation of antinociception and a variety of behavioral states like addiction, anxiety, and depression. It is composed of four G-protein coupled receptors: kappa (KOR), mu (MOR), delta (DOR), and nociception (NOR), that are expressed throughout both the peripheral and central nervous system (CNS). Salvinorin A, the main active ingredient in the hallucinogenic plant Salvia divinorum, is one of the most potent, naturally occurring opioid agonists with high selectivity and affinity for KOR. It has the potential to be beneficial in therapy of various CNS disorders. Due to its strong hallucinating effects, salvinorin A has never advanced to clinical trials, but has been used as an important prototype for the development of related drug candidates. Our lab aims to use the Salvinorin scaffold to develop novel compounds to probe the opioid system.
Mentor: Dr. Mahmoud ElSohly & Dr. Mohamed Radwan
Introduction of hydrophilic functionalities into the cannabidiol molecule to ameliorate its lipophilicity for enhancement of its antiepileptic utility
This application is to investigate, primarily, the applicability of singlet oxygen photooxygenation in the introduction of hydrophilic regions in the molecule of cannabidiol (CBD) in order to diminish its excessive lipophilicity. On June 25, 2018, the FDA approved CBD as a treatment for two rare forms of childhood epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. CBD has been reported to possess a wide range of potential therapeutic utilities for several illnesses, including seizure-suppression, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, epilepsy, neuroprotection, depression, as an anxiolytic and as an analgesic. In addition to possessing central effects, CBD exhibits therapeutically beneficial peripheral actions such as anti-inflammatory. Despite the many pharmacological potentials of CBD, its drawbacks have limited its applications in therapeutics. Excessive Lipophilicity has been persistently a major challenge for drug development. The major drawbacks of CBD include: low water solubility, poor absorption, low oral bioavailability (approximately 10%), very high plasma protein binding (>99%), and significant first-pass metabolism. To enhance the absorption and bioavailability of CBD, its excessive lipophilicity should be counteracted by introduction of a hydrophilic region in the CBD molecule. This can be accomplished by Singlet oxygen photooxygenation of CBD. Singlet oxygen photooxygenation, an established reaction, unlike other oxidation methodologies, it results in formation of multiple products with a range of oxygenated/hydroxylated functional groups in a single step. Varying the reaction conditions, including using different photosensitizers, solvents, reaction time, irradiation with UV or incandescent light has resulted in the formation of vicinal diol derivatives, quinones, ethers, and oxides, beside the typical allylic hydroperoxides. The lipophilicity of these products (expressed as logP) will be determined in silico and experimentally, for validation, using HPLC-MS methods. Compounds with logP <5.0, will be advanced for further evaluation. The selected derivatives will be examined for potential affinity to the CB1 receptor, to exclude compounds with psychoactivity. The selected derivatives will be tested in the in vivo Genetic Zebrafish model of epilepsy (Dravet). The second year of the proposed project will be utilized for optimization and scaling up of the selected CBD derivatives, based on the work done in the first year, and will be followed by examination of their antiepileptic effects, utilizing in vivo models. The effect of reducing lipophilicity on the GIT. Permeability will be tested using in vitro models, e.g. the Caco-2 cell assay.
Mentor: Dr. Marc Kaufman
Natural Neuroendocrine Modulators for HIV-Accelerated Aging
Given the advent of combined antiretroviral therapeutics (cART), the population infected with human immunodeficiency virus type 1 (HIV-1) is aging. In the U.S. alone, nearly 700,000 HIV+ individuals are over the age of 50. Compared to those that are uninfected, these individuals are at risk for an accelerated aging phenotype characterized by sooner transition to the climacteric (post-menopause in women, andropause in men) and predisposition to vasomotor symptoms, cognitive deficits, neuropsychiatric disorder, and age-related frailty (i.e. osteoporosis, muscle weakness, gait/motor aberrations). Symptom burden is typically worse among women compared to men, particularly those reporting amenorrhea vs. those still naturally-cycling.
The mechanisms underlying HIV-accelerated aging are not known and cART does not ameliorate symptomology. We and others have identified the HIV-1 trans-activator of transcription (Tat) protein to be one the most neurotoxic soluble proteins secreted by the virus. Many neurological symptoms may occur downstream of HIV-1 Tat actions, cART does not target Tat, and pregnane steroids (progesterone and allopregnanolone) can ameliorate some Tat-mediated behavioral and CNS pathology. We propose to investigate HIV-accelerated aging in a mouse model of neuroHIV that conditionally-expresses Tat protein. Aged (12-15 mos old) male and female Tat-expressing mice (and their non-Tat-expressing counterparts) will be stratified by endocrine aging status as having maintained reproductive capacity (pre-estropausal) or having transitioned to reproductive senescence (post-estropausal). In Aim 1, mice will be assessed for neuroendocrine measures (pregnenolone and allopregnanolone content) via UPLC/MS, neurocognitive/neuropsychiatric and nociceptive behavior, and indices of frailty. In Aim 2, mice will (or will not) receive a pharmacological enhancer of natural neurosteroids or allopregnanolone and will be assessed for behavior as described in Aim 1, brain morphometry (region gray-matter density, white matter abnormality, and ventricular volume) via MRI, neuronal morphology via Golgi-Kopsch staining, and frailty.
We anticipate HIV-1 Tat exposure to confer vulnerability to an accelerated aging phenotype (particularly in post-estropausal females) and natural neurosteroid enhancement to ameliorate related comorbidities.
Voucher Program
Eligibility: All University of Mississippi faculty members are eligible to participate in the Pilot Project Program. Preference will be given to junior investigators who are in the early stages of their career. Those who have not yet received a major program grant (e.g. an R01) award are also encouraged to apply for the PPP. Past graduates from the COBRE-NPN program, who do not serve as mentors, may also compete for the projects but will have to present a compelling rationale to be considered for funding.
Project areas that fall within the scientific focus of the CORE-NPN include but are not limited to:
-
- Basic science research: biological evaluation of natural products as agents possessing neuropharmacologic effects.
- Translational science research: Translating basic science discoveries from animal models to human subjects.
- Commercialization (Entrepreneurial development): The advancement of research discoveries from the bench to the commercial sector.
Criteria
Criteria for evaluation of COBRE application:
-
- Initial evaluation will be done based on the cover page and specific aims submitted by all applicants and will be done by the PPP Review Committee and the EAC. The rubric for narrowing the pool is based primarily on:
- Relevance to the COBRE-NPN research focus
- Clarity of specific aims and scientific merit
- Junior Investigator status
- Proposed COBRE-NPN core utilization
- Strategic vision for leveraging PPP results into future R-type NIH funding
- Initial evaluation will be done based on the cover page and specific aims submitted by all applicants and will be done by the PPP Review Committee and the EAC. The rubric for narrowing the pool is based primarily on:
-
- A maximum of eight applicants will be selected. These applicants will be invited to submit a full-proposal, in R01-format, and will then participate in a Shark Tank-type meeting wherein a 10-minute presentation of the proposal is given to the PPP Review Committee who in turn provide the applicant feedback and guidance.
- Applicants will then submit a revised (A1-like) proposal to be forwarded to the EAC for final evaluation.
- The EAC will select up to five finalists pending NIGMS approval.
- Proposals will be prioritized for funding based on scientific merit, thematic research focus, career stage, collaboration teams, and the perceived likelihood of publications and competitive grant applications generated from work.
General Terms and Conditions
General Terms and Conditions of CORE-NPN COBRE Research Pilot Project Program:
-
- COBRE funds will not be released until NIGMS approves the project.
- PI or Co-I salary will not be supported. Funds may be used to support purchase of materials and supplies and for laboratory personnel salaries.
- At least 30 percent of the funds should be used for services obtained through one or both Cores (Chemistry and DMPK and/or Neuropharmacology Core).
- Pilot Project Investigators will be required to deliver a presentation every quarter on their progress.
- The investigator will be required to submit at least one abstract for presentation at a national research meeting and to submit one manuscript for peer-reviewed publication acknowledging COBRE and core support, by the end of year 1 of funding.
- Recipients will be required to submit an investigator-initiated NIH R01 grant application by the end of the second year of COBRE support. If investigator obtains external support, COBRE support will be discontinued if the COBRE supported project is significantly similar to the newly funded project.
- Investigators will be expected to present their research findings to the EAC during their annual review.
- The younger scientists, pre-R01 awardee, and assistant professors will be required to identify at least one senior faculty member to serve as their mentor. The more senior scientists at the associate or full professor rank will have the option of selecting a senior mentor to facilitate the development of their project.
- Recipients will present their work at the annual Core Research Day.
Prospective applicants with questions about eligibility, program details, application process or requirements are encouraged to contact Dr. Kristie Willett (662-915-6691; kwillett@olemiss.edu).
Current Pilot Projects

Project Title: 5HT2A agonist microdoses for pharmacotherapy of treatment-resistant depression
PI: Glenn Walker
Mentor: Dr. David Puleo
There is an urgent unmet medical need to develop therapeutic options for the ~50% of depression patients suffering from treatment-resistant depression, which is difficult to treat with existing psycho- and pharmacotherapeutic options. Classical psychedelics, such as the 5HT2A agonists lysergic acid diethylamide (LSD) and psilocybin, have re-emerged as a treatment paradigm for depression. Recent clinical trials highlight the potential effectiveness of 5HT2A agonists to improve mood and psychotherapeutic growth in treatment resistant depression patients, even in those who have failed a median of 4 previous medications in their lifetime. Accordingly, the FDA recently granted “Breakthrough Therapy” designation to psilocybin for
treatment-resistant depression. The improvements associated with 5HT2A agonist pharmacotherapy typically last for weeks to months, a marked increase relative to ketamine, but many patients find their symptoms recur and need repeat administration. ‘Microdosing’ – ingesting subperceptual doses of psychedelics every other (Q48) or every third day (Q72) to improve mood and/or creativity – represents an alternative, potentially long-term therapy for treatment-resistant depression patients. Microdosing has become prominent in the lay public, with recent scientific studies corroborating anecdotal claims that it improves mood, energy levels, social connectedness, and decreases craving for addictive substances. However, there are a gamut of practical barriers that stymie further investigation of microdosing 5HT2A agonists, including: low compliance with the complicated dosing regimen, high risk of diversion of controlled substances, and difficulty and cost administering the long-term treatment regimens in controlled settings. Here, we propose to overcome the above limitations by developing a bioresorbable microdosing implant (MDI) that enables long-term, intermittent delivery of sub-perceptual doses of 5HT2A agonists. The MDIs are fabricated by layering surface eroding polymer films composed of Cellulose Acetate Phthalate (CAP) and Pluronic F-127 (P) polymers.
These films can be layered to achieve sequential and/or intermittent drug release and the drug release rate and time between doses can be tuned by the film thickness. We will use the enabling CAPP film technology to test the hypothesis that long-term and consistent Q72 microdosing of 5HT2A agonists can be achieved by alternating 5HT2A agonist-loaded and unloaded CAPP films.
 Project Title: Microbiome Drivers of Cyanobacterial Neuroactive Natural Product Production.
Project Title: Microbiome Drivers of Cyanobacterial Neuroactive Natural Product Production.
Mentor: Vivian Hook
Cyanobacteria are an ancient clade of bacteria which are globally distributed, photosynthetic, and diverse. While they have a major role in the Earth’s carbon and nitrogen cycles, they are also of interest because genomics has shown they often bear multiple natural products biosynthetic pathways. The literature on the isolation of these cyanobacterial secondary metabolites is extensive and some of these compounds have been brought into clinical trials, but less understood is the ecological role of these compounds and how a given cyanobacterium regulates the production of multiple biosynthetic pathways. This project aims to tackle both
of these unanswered questions by using modifications of the cyanobacterial heterotrophic microbiome to change the cyanobacterial metabolome. Stressors such as temperature, salinity, and UV irradiation will drive changes in the microbiome, along with the use of antibiotics to eliminate susceptible heterotrophs from the microbiome. Cyanobacterial hosts that have had their microbiome community reduced in these experiments can then be reinoculated with individual heterotrophs isolated from the original axenic culture to see if the heterotroph can reestablish its symbiosis and elicit natural products from the host. Monitoring of
the cultures will use metagenomic sequencing, LCMS metabolomic screening, and bioassay for neuroactivity in the COBRE Neuropharmacology Core. The combined data sets will reveal not only changes in the microbiome, or the production of novel bioactive metabolites, but allow these observations to be correlated. Within the objective of driving metabolic change in the host through the microbiome community, the LCMS screening will show those changes in the metabolome and the metagenomic sequencing will reveal what heterotrophs might be responsible for those changes. When the metabolome of a cyanobacterium changes under different growth conditions as observed by LCMS comparing extracts from those two conditions by bioassay will be used to assign the difference in chemical composition to differences in the bioassay result. These assignments will then be validated by isolating and testing the natural product.
 Project Title: Plant Essential Oil Components that Interact with the Cannabinoid Receptor CB2
Project Title: Plant Essential Oil Components that Interact with the Cannabinoid Receptor CB2
PI: Dr. Xing-Cong Li
Mentor: Dr. Samir Ross
The human endocannabinoid system is a biological signaling system in the brain that regulates numerous physiological and cognitive processes. It is a promising therapeutic target for many neurological diseases. In particular, cannabinoid receptor type 2 (CB2) that is highly expressed in peripheral immune tissues and to a lesser extent in neurons and microglial cells plays a critical role in pain, anti-inflammation, and neuroprotection. We have identified seven plant-derived essential oils (EOs) possessing potent CB2 binding affinity, which may explain the medicinal properties of these EOs in aromatherapy to relieve neurological and psychiatric disorders. A preliminary chemical analysis showed that these seven EOs contain new chemotypes of cannabinoid CB2 ligands. The proposed research seeks to identify these unique phytocannabinoids that may be utilized as lead compounds for the development of a new treatment to manage depression, a major psychiatric condition affecting hundreds of millions in modern society. A novel natural products drug discovery approach will be employed to achieve this goal in an efficient way. Specifically, we will perform gas chromatography-mass spectrometry analysis to quickly determine candidate compound structures in the seven EOs. We will then conduct a virtual screening to rapidly prioritize possible active compounds. These
compounds will be acquired from commercial sources or by isolation and chemical synthesis to test their CB2 affinity, in vitro pharmacological activities, and in vivo anti-depressant efficacy in a mouse model. This approach is innovative because it reduces the conventional natural products drug discovery requirement for tremendous efforts in dereplication, isolation, and structural elucidation. In addition, a lead compound will be selected for structural optimization. A structure-based approach will be utilized to design and synthesize novel compounds with improved CB2 affinity and druggable properties. Successful completion of the project will result in the identification of structurally diverse naturally occurring cannabinoids, synthetic cannabinoids with improved CB2 affinity, and one or two lead compounds with confirmed in vivo anti-depressant efficacy, which will lay a solid foundation for future drug development.
 Project Title: Could vitamin E acetate oil be responsible for the marijuana vaping illnesses?
Project Title: Could vitamin E acetate oil be responsible for the marijuana vaping illnesses?
PI: Mona H. Haron
Mentors: Dr. Samir Ross and Dr. Shabana Khan
The outbreak of lung injury associated with the use of E-cigarette or vaping products (EVALI) on the summer of 2019 brought to the spot light the possible dangerous of smoking/inhaling cannabis/marijuana and tetrahydrocannabinol (THC). As of February 18, 2020, a total of 2,807 hospitalized EVALI cases or deaths have been reported to CDC from all 50 states, the District of Columbia, and two U.S. territories (Puerto Rico and U.S. Virgin Islands). Sixty-eight deaths have been confirmed in 29 states and the District of Columbia. Vitamin E oil in marijuana and tetrahydrocannabinol (THC) vape is currently a key focus of investigators looking into vape-associated lung injures. Human use of THC is usually via inhalation of cannabis combustion products (smoking) for its psycho-activity. Although cannabis is illegal at the federal level in the United States, products e.g. marijuana vape-pens are readily available for purchase on the Internet, at head shops, and dispensaries in states where medical or recreation cannabis is legal. Vaporizable hash oil and THC waxes were reported to deliver higher THC concentrations than combustible cannabis by 4–30 times; causing stronger exposure experience to THC. Several cases of severe illnesses related to vaping, in patients ranging in age from 15 to 46 were reported where in each case; the patient had used “at least one”
cannabis-related product. In clinician’s reports many of the illnesses was linked to people using marijuana vaping. Vitamin E oil was found as diluent in most of the marijuana but not the nicotine-based vaping products that were tested in relation to vaping illness outbreak. These findings made vitamin E acetate an agent of interest in causing those illnesses. Vitamin E is a dietary supplement that is used in some skin products with minimal side effects but its health effects are not clear when smoked alone or with THC. The rational of this study is, since in some of the cases the duration between the patients starting vaping and the onset of symptoms is very short, it is possible that the illnesses are happening due to a local (direct) effect on the lungs as well as a central (indirect) effect via cannabinoid receptors (CB) in the brain. Vitamin
E oil presence with THC in some vaping products could be causing very high levels of THC exposure by the brain via enhancing it is solubility and increasing its bioavailability. This could be predisposing and contributing to the onset of the reported illnesses. The objective of this study is determining the brain, heart and lung bioavailability and CB1 and CB2 binding affinities of THC, vitamin E acetate and their combination in concentrations similar to that present in marijuana vaping products.
 Project Title: Discovery of potential disruptors of CB1R:5HT2AR heteromer to mitigate the THC-induced cognitive impairment
Project Title: Discovery of potential disruptors of CB1R:5HT2AR heteromer to mitigate the THC-induced cognitive impairment
PI: Amar G. Chittiboyina, Ph.D., FRSc
Key members: Drs. Pankaj Pandey, Osman Galal
Mentor: Professor Robert J. Doerksen
Lately, cannabis (Cannabis sativa) consumption for medical and recreational purposes has been hotly debated, and the passage of the 2018 Farm Bill on industrial hemp (C. sativa with <0.3% THC) allowed consumers to readily access such materials for the management of various medical symptoms including neuropathic pain. Cannabis contains more than 600 phytoconstituents; among them, ~120 constituents are recognized as cannabinoids and another 120+ compounds as terpenes. Many of these constituents are recognized to have deleterious and(or) potential therapeutic effects, which are often mediated through the endogenous cannabinoid system. THC, the main psychoactive compound in cannabis, acts as a cannabinoid receptor type 1 (CB1) agonist and is associated with potential therapeutic applications, including analgesic, anxiolytic-like, and neuroprotective attributes. However, in addition to THC’s abuse potential, THC also induces numerous undesirable behavioral effects, including memory impairment and anxiogenic effects. Serotonin 2A receptors (5HT2A) are reported to modulate THC’s effects on acute amnesia, anxiety, social interaction and hence limited cannabis use and effectiveness as an analgesic. However, data for mice lacking 5HT2A suggest that the analgesic and amnesic effects of THC are independent of each other; that is, THC-induced amnesia is absent in the mutant mice, yet THC still promotes analgesia in these animals. This provides a welcome opportunity to target a receptor population that produces and(or) regulates the deleterious effects of THC. Indeed, recent data suggest that pharmacological targeting of the unique CB1:5HT2A heteromer may be exploited as a potential target to overcome THC-induced side effects.
We propose that some of the minor cannabinoids and terpenes, including CBD and limonene, can bind and regulate both CB1 and 5HT2A, which would allow us to disrupt the formation of the CB1:5HT2A heteromer, and afford the potential to dissociate the deleterious effects induced by THC from its beneficial antinociceptive properties. To this end, we will implement structure-based drug design techniques for the construction and validation of the CB1:5HT2A heteromer complex; identification of “hot-spot residues” at the interface of the heteromer, and perform protein-target-based virtual screening of public databases and all phytoconstituents of cannabis to discover potential disruptors/inhibitors of the CB1:5HT2A heteromer.
Based on these structural studies, the role of identified potential disruptors, including naturally occurring components of cannabis (cannabinoids, terpenes, and others) for their inhibitory effects on heteromer, will be explored and further validated with behavioral studies on mice to separate antinociceptive effects from cognitive effects induced by THC.
Past Pilot Projects
 Dr. Alberto Jose Del Arco Gonzalez
Dr. Alberto Jose Del Arco Gonzalez
From Stress to Drug Addiction: The Neuronal Networks that Underlie Vulnerability to Drug Abuse
The repeated exposure to stressful experiences makes subjects more vulnerable to develop drug abuse. Vulnerability to drug abuse is associated with the abnormal function of the brain reward system which promotes drug-seeking behavior and enhances the reinforcing effects of drugs (drug sensitization). However, how stress alters the reward system to transform a healthy brain into a vulnerable brain is poorly understood. In preliminary experiments I find that exposing rats to repetitive intermittent social defeat stress (RISD) alters reward-seeking behavior. In line with these findings, previous studies have shown that RISD produces cocaine sensitization and promotes cocaine self-administration. Yet the neuronal networks that explain these behaviors are still unclear. The main goal of this project is to identify the neuronal networks in the brain that lead to abnormal reward-seeking behavior and enhance the effects of drugs during repetitive exposure to stress. Specifically, I will record in vivo neuronal activity in the prefrontal cortex (PFC) and the ventral tegmental area (VTA) since both areas of the brain process rewarding stimuli and regulate the response to stress.
The central hypothesis of this proposal is that the repetitive exposure to social stress changes how neurons respond to (i.e. encode) reward-seeking cues in the PFC and the VTA. These neuronal changes alter reward-seeking behavior and enhance the reinforcing effects of drugs, making subjects more vulnerable to develop drug addiction. To test this hypothesis:
Specific Aim 1 will determine the neuronal response to reward-seeking cues during social stress. Specific Aim 2 will determine whether cocaine enhances the neuronal response to reward-seeking cues after social stress.
Importantly, this project will take advantage of the Neuropharmacology CORE-NPN to carry out a multilevel experimental design in which the neuronal activity in the brain after stress is associated with changes in other behavioral and molecular parameters such as motor activity (open field test), rewarding effects (conditioned place preference test), anxiety behavior (plus maze test) and the activity of stress hormones (I.e. corticotropin-releasing factor, corticosterone) in the brain (immunohistochemistry and western blot analysis). Furthermore, the results of this research will help to develop more competitive grant applications to secure NIH R01 funding and promote collaborations between our campus and the Medical School.

Mentor: Dr. Samir Ross & Dr. Larry Walker
Novel and Selective CB2 Agonist/Inverse Agonist for Targeting Neurodenerative Disorders
The cannabinoid receptor subtype 2 (CB2) is emerging as a potential target for the development of new therapeutic approaches in several neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). CB2 is expressed by peripheral immune cells and mediates the immunosuppressive effects of cannabinoids and endocannabinoids. Recently, we have developed a new class of selective CB2 ligands as lead compounds for neuroinflammatory and neurodegenerative disorders. Pyrrolo[2,1-c][1,4] benzodiazepines (PBDs) are a class of natural products obtained from various actinomycetes which have both anti-tumor and antibiotic activities. Closer inspection of the newly designed PBD tricyclic skeleton in analogy to the natural CB ligands backbone such as THC and CBN, has sparked an innovative interest in this under explored class of the compounds as a potential source of CB2 selective ligands. We hypothesize that PBD analogs should have the proper configuration that gives the molecules a right-handed twist which will offer the appropriate three-dimensional conformation to fit snugly within the active site of CB receptors, enabling them to interfere with the endocannabinoid signaling system. Interestingly, computational studies and theoretical binding affinities of six selected (S,E)-11-[2-(arylmethylene)hydrazono]-PBD analogs, have revealed the presence of a selective tight binding attraction towards CB1 and CB2 which have been confirmed via testing. Intriguingly, one of the synthesized compounds showed a selective binding affinity towards CB2 (Ki = 8.4 nM) when compared to CB1 (Ki =65.4 nM). Our finding suggested that introduction of a halogen on the aromatic ring promotes the selectivity of CB2-ligand interaction while introducing a polar side-chain on the aromatic ring escalates the selectivity towards CB1 receptor. This in turn motivates us to move forward with optimizing our scheme for amplifying the selectivity through the generation of twenty four (S,E)-11-[2-(arylmethylene)hydrazono]-PBD analogs supported by computer-based docking studies and the drug-likeness properties via computational calculation of ADMET and physicochemical properties. The generated ligands will be evaluated for their CB receptor binding and GTP–[35S] assays as well as neuronal viability assessment in CORE-NPN. The most potent analogs will be tested in Alzheimer’s mouse model, J20 (PDGF-APPSw,Ind). The outcome of these studies has the potential to lead to potent therapeutic agents for neurodegenerative diseases and will open the window for future exploratory funding opportunities.
Development of Novel Salvinorin Compounds with 6, 5-fused Rings at C-2
The opioid system plays a crucial role in the modulation of antinociception and a variety of behavioral states like addiction, anxiety, and depression. It is composed of four G-protein coupled receptors: kappa (KOR), mu (MOR), delta (DOR), and nociception (NOR), that are expressed throughout both the peripheral and central nervous system (CNS). Salvinorin A, the main active ingredient in the hallucinogenic plant Salvia divinorum, is one of the most potent, naturally occurring opioid agonists with high selectivity and affinity for KOR. It has the potential to be beneficial in therapy of various CNS disorders. Due to its strong hallucinating effects, salvinorin A has never advanced to clinical trials, but has been used as an important prototype for the development of related drug candidates. Our lab aims to use the Salvinorin scaffold to develop novel compounds to probe the opioid system.
Mentor: Dr. Mahmoud ElSohly & Dr. Mohamed Radwan
Introduction of hydrophilic functionalities into the cannabidiol molecule to ameliorate its lipophilicity for enhancement of its antiepileptic utility
This application is to investigate, primarily, the applicability of singlet oxygen photooxygenation in the introduction of hydrophilic regions in the molecule of cannabidiol (CBD) in order to diminish its excessive lipophilicity. On June 25, 2018, the FDA approved CBD as a treatment for two rare forms of childhood epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. CBD has been reported to possess a wide range of potential therapeutic utilities for several illnesses, including seizure-suppression, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, epilepsy, neuroprotection, depression, as an anxiolytic and as an analgesic. In addition to possessing central effects, CBD exhibits therapeutically beneficial peripheral actions such as anti-inflammatory. Despite the many pharmacological potentials of CBD, its drawbacks have limited its applications in therapeutics. Excessive Lipophilicity has been persistently a major challenge for drug development. The major drawbacks of CBD include: low water solubility, poor absorption, low oral bioavailability (approximately 10%), very high plasma protein binding (>99%), and significant first-pass metabolism. To enhance the absorption and bioavailability of CBD, its excessive lipophilicity should be counteracted by introduction of a hydrophilic region in the CBD molecule. This can be accomplished by Singlet oxygen photooxygenation of CBD. Singlet oxygen photooxygenation, an established reaction, unlike other oxidation methodologies, it results in formation of multiple products with a range of oxygenated/hydroxylated functional groups in a single step. Varying the reaction conditions, including using different photosensitizers, solvents, reaction time, irradiation with UV or incandescent light has resulted in the formation of vicinal diol derivatives, quinones, ethers, and oxides, beside the typical allylic hydroperoxides. The lipophilicity of these products (expressed as logP) will be determined in silico and experimentally, for validation, using HPLC-MS methods. Compounds with logP <5.0, will be advanced for further evaluation. The selected derivatives will be examined for potential affinity to the CB1 receptor, to exclude compounds with psychoactivity. The selected derivatives will be tested in the in vivo Genetic Zebrafish model of epilepsy (Dravet). The second year of the proposed project will be utilized for optimization and scaling up of the selected CBD derivatives, based on the work done in the first year, and will be followed by examination of their antiepileptic effects, utilizing in vivo models. The effect of reducing lipophilicity on the GIT. Permeability will be tested using in vitro models, e.g. the Caco-2 cell assay.
Mentor: Dr. Marc Kaufman
Natural Neuroendocrine Modulators for HIV-Accelerated Aging
Given the advent of combined antiretroviral therapeutics (cART), the population infected with human immunodeficiency virus type 1 (HIV-1) is aging. In the U.S. alone, nearly 700,000 HIV+ individuals are over the age of 50. Compared to those that are uninfected, these individuals are at risk for an accelerated aging phenotype characterized by sooner transition to the climacteric (post-menopause in women, andropause in men) and predisposition to vasomotor symptoms, cognitive deficits, neuropsychiatric disorder, and age-related frailty (i.e. osteoporosis, muscle weakness, gait/motor aberrations). Symptom burden is typically worse among women compared to men, particularly those reporting amenorrhea vs. those still naturally-cycling.
The mechanisms underlying HIV-accelerated aging are not known and cART does not ameliorate symptomology. We and others have identified the HIV-1 trans-activator of transcription (Tat) protein to be one the most neurotoxic soluble proteins secreted by the virus. Many neurological symptoms may occur downstream of HIV-1 Tat actions, cART does not target Tat, and pregnane steroids (progesterone and allopregnanolone) can ameliorate some Tat-mediated behavioral and CNS pathology. We propose to investigate HIV-accelerated aging in a mouse model of neuroHIV that conditionally-expresses Tat protein. Aged (12-15 mos old) male and female Tat-expressing mice (and their non-Tat-expressing counterparts) will be stratified by endocrine aging status as having maintained reproductive capacity (pre-estropausal) or having transitioned to reproductive senescence (post-estropausal). In Aim 1, mice will be assessed for neuroendocrine measures (pregnenolone and allopregnanolone content) via UPLC/MS, neurocognitive/neuropsychiatric and nociceptive behavior, and indices of frailty. In Aim 2, mice will (or will not) receive a pharmacological enhancer of natural neurosteroids or allopregnanolone and will be assessed for behavior as described in Aim 1, brain morphometry (region gray-matter density, white matter abnormality, and ventricular volume) via MRI, neuronal morphology via Golgi-Kopsch staining, and frailty.
We anticipate HIV-1 Tat exposure to confer vulnerability to an accelerated aging phenotype (particularly in post-estropausal females) and natural neurosteroid enhancement to ameliorate related comorbidities.

Voucher Program
 Dr. Alberto Jose Del Arco Gonzalez
Dr. Alberto Jose Del Arco Gonzalez
From Stress to Drug Addiction: The Neuronal Networks that Underlie Vulnerability to Drug Abuse
The repeated exposure to stressful experiences makes subjects more vulnerable to develop drug abuse. Vulnerability to drug abuse is associated with the abnormal function of the brain reward system which promotes drug-seeking behavior and enhances the reinforcing effects of drugs (drug sensitization). However, how stress alters the reward system to transform a healthy brain into a vulnerable brain is poorly understood. In preliminary experiments I find that exposing rats to repetitive intermittent social defeat stress (RISD) alters reward-seeking behavior. In line with these findings, previous studies have shown that RISD produces cocaine sensitization and promotes cocaine self-administration. Yet the neuronal networks that explain these behaviors are still unclear. The main goal of this project is to identify the neuronal networks in the brain that lead to abnormal reward-seeking behavior and enhance the effects of drugs during repetitive exposure to stress. Specifically, I will record in vivo neuronal activity in the prefrontal cortex (PFC) and the ventral tegmental area (VTA) since both areas of the brain process rewarding stimuli and regulate the response to stress.
The central hypothesis of this proposal is that the repetitive exposure to social stress changes how neurons respond to (i.e. encode) reward-seeking cues in the PFC and the VTA. These neuronal changes alter reward-seeking behavior and enhance the reinforcing effects of drugs, making subjects more vulnerable to develop drug addiction. To test this hypothesis:
Specific Aim 1 will determine the neuronal response to reward-seeking cues during social stress. Specific Aim 2 will determine whether cocaine enhances the neuronal response to reward-seeking cues after social stress.
Importantly, this project will take advantage of the Neuropharmacology CORE-NPN to carry out a multilevel experimental design in which the neuronal activity in the brain after stress is associated with changes in other behavioral and molecular parameters such as motor activity (open field test), rewarding effects (conditioned place preference test), anxiety behavior (plus maze test) and the activity of stress hormones (I.e. corticotropin-releasing factor, corticosterone) in the brain (immunohistochemistry and western blot analysis). Furthermore, the results of this research will help to develop more competitive grant applications to secure NIH R01 funding and promote collaborations between our campus and the Medical School.

Mentor: Dr. Samir Ross & Dr. Larry Walker
Novel and Selective CB2 Agonist/Inverse Agonist for Targeting Neurodenerative Disorders
The cannabinoid receptor subtype 2 (CB2) is emerging as a potential target for the development of new therapeutic approaches in several neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). CB2 is expressed by peripheral immune cells and mediates the immunosuppressive effects of cannabinoids and endocannabinoids. Recently, we have developed a new class of selective CB2 ligands as lead compounds for neuroinflammatory and neurodegenerative disorders. Pyrrolo[2,1-c][1,4] benzodiazepines (PBDs) are a class of natural products obtained from various actinomycetes which have both anti-tumor and antibiotic activities. Closer inspection of the newly designed PBD tricyclic skeleton in analogy to the natural CB ligands backbone such as THC and CBN, has sparked an innovative interest in this under explored class of the compounds as a potential source of CB2 selective ligands. We hypothesize that PBD analogs should have the proper configuration that gives the molecules a right-handed twist which will offer the appropriate three-dimensional conformation to fit snugly within the active site of CB receptors, enabling them to interfere with the endocannabinoid signaling system. Interestingly, computational studies and theoretical binding affinities of six selected (S,E)-11-[2-(arylmethylene)hydrazono]-PBD analogs, have revealed the presence of a selective tight binding attraction towards CB1 and CB2 which have been confirmed via testing. Intriguingly, one of the synthesized compounds showed a selective binding affinity towards CB2 (Ki = 8.4 nM) when compared to CB1 (Ki =65.4 nM). Our finding suggested that introduction of a halogen on the aromatic ring promotes the selectivity of CB2-ligand interaction while introducing a polar side-chain on the aromatic ring escalates the selectivity towards CB1 receptor. This in turn motivates us to move forward with optimizing our scheme for amplifying the selectivity through the generation of twenty four (S,E)-11-[2-(arylmethylene)hydrazono]-PBD analogs supported by computer-based docking studies and the drug-likeness properties via computational calculation of ADMET and physicochemical properties. The generated ligands will be evaluated for their CB receptor binding and GTP–[35S] assays as well as neuronal viability assessment in CORE-NPN. The most potent analogs will be tested in Alzheimer’s mouse model, J20 (PDGF-APPSw,Ind). The outcome of these studies has the potential to lead to potent therapeutic agents for neurodegenerative diseases and will open the window for future exploratory funding opportunities.
Development of Novel Salvinorin Compounds with 6, 5-fused Rings at C-2
The opioid system plays a crucial role in the modulation of antinociception and a variety of behavioral states like addiction, anxiety, and depression. It is composed of four G-protein coupled receptors: kappa (KOR), mu (MOR), delta (DOR), and nociception (NOR), that are expressed throughout both the peripheral and central nervous system (CNS). Salvinorin A, the main active ingredient in the hallucinogenic plant Salvia divinorum, is one of the most potent, naturally occurring opioid agonists with high selectivity and affinity for KOR. It has the potential to be beneficial in therapy of various CNS disorders. Due to its strong hallucinating effects, salvinorin A has never advanced to clinical trials, but has been used as an important prototype for the development of related drug candidates. Our lab aims to use the Salvinorin scaffold to develop novel compounds to probe the opioid system.
Mentor: Dr. Mahmoud ElSohly & Dr. Mohamed Radwan
Introduction of hydrophilic functionalities into the cannabidiol molecule to ameliorate its lipophilicity for enhancement of its antiepileptic utility
This application is to investigate, primarily, the applicability of singlet oxygen photooxygenation in the introduction of hydrophilic regions in the molecule of cannabidiol (CBD) in order to diminish its excessive lipophilicity. On June 25, 2018, the FDA approved CBD as a treatment for two rare forms of childhood epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. CBD has been reported to possess a wide range of potential therapeutic utilities for several illnesses, including seizure-suppression, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, epilepsy, neuroprotection, depression, as an anxiolytic and as an analgesic. In addition to possessing central effects, CBD exhibits therapeutically beneficial peripheral actions such as anti-inflammatory. Despite the many pharmacological potentials of CBD, its drawbacks have limited its applications in therapeutics. Excessive Lipophilicity has been persistently a major challenge for drug development. The major drawbacks of CBD include: low water solubility, poor absorption, low oral bioavailability (approximately 10%), very high plasma protein binding (>99%), and significant first-pass metabolism. To enhance the absorption and bioavailability of CBD, its excessive lipophilicity should be counteracted by introduction of a hydrophilic region in the CBD molecule. This can be accomplished by Singlet oxygen photooxygenation of CBD. Singlet oxygen photooxygenation, an established reaction, unlike other oxidation methodologies, it results in formation of multiple products with a range of oxygenated/hydroxylated functional groups in a single step. Varying the reaction conditions, including using different photosensitizers, solvents, reaction time, irradiation with UV or incandescent light has resulted in the formation of vicinal diol derivatives, quinones, ethers, and oxides, beside the typical allylic hydroperoxides. The lipophilicity of these products (expressed as logP) will be determined in silico and experimentally, for validation, using HPLC-MS methods. Compounds with logP <5.0, will be advanced for further evaluation. The selected derivatives will be examined for potential affinity to the CB1 receptor, to exclude compounds with psychoactivity. The selected derivatives will be tested in the in vivo Genetic Zebrafish model of epilepsy (Dravet). The second year of the proposed project will be utilized for optimization and scaling up of the selected CBD derivatives, based on the work done in the first year, and will be followed by examination of their antiepileptic effects, utilizing in vivo models. The effect of reducing lipophilicity on the GIT. Permeability will be tested using in vitro models, e.g. the Caco-2 cell assay.
Mentor: Dr. Marc Kaufman
Natural Neuroendocrine Modulators for HIV-Accelerated Aging
Given the advent of combined antiretroviral therapeutics (cART), the population infected with human immunodeficiency virus type 1 (HIV-1) is aging. In the U.S. alone, nearly 700,000 HIV+ individuals are over the age of 50. Compared to those that are uninfected, these individuals are at risk for an accelerated aging phenotype characterized by sooner transition to the climacteric (post-menopause in women, andropause in men) and predisposition to vasomotor symptoms, cognitive deficits, neuropsychiatric disorder, and age-related frailty (i.e. osteoporosis, muscle weakness, gait/motor aberrations). Symptom burden is typically worse among women compared to men, particularly those reporting amenorrhea vs. those still naturally-cycling.
The mechanisms underlying HIV-accelerated aging are not known and cART does not ameliorate symptomology. We and others have identified the HIV-1 trans-activator of transcription (Tat) protein to be one the most neurotoxic soluble proteins secreted by the virus. Many neurological symptoms may occur downstream of HIV-1 Tat actions, cART does not target Tat, and pregnane steroids (progesterone and allopregnanolone) can ameliorate some Tat-mediated behavioral and CNS pathology. We propose to investigate HIV-accelerated aging in a mouse model of neuroHIV that conditionally-expresses Tat protein. Aged (12-15 mos old) male and female Tat-expressing mice (and their non-Tat-expressing counterparts) will be stratified by endocrine aging status as having maintained reproductive capacity (pre-estropausal) or having transitioned to reproductive senescence (post-estropausal). In Aim 1, mice will be assessed for neuroendocrine measures (pregnenolone and allopregnanolone content) via UPLC/MS, neurocognitive/neuropsychiatric and nociceptive behavior, and indices of frailty. In Aim 2, mice will (or will not) receive a pharmacological enhancer of natural neurosteroids or allopregnanolone and will be assessed for behavior as described in Aim 1, brain morphometry (region gray-matter density, white matter abnormality, and ventricular volume) via MRI, neuronal morphology via Golgi-Kopsch staining, and frailty.
We anticipate HIV-1 Tat exposure to confer vulnerability to an accelerated aging phenotype (particularly in post-estropausal females) and natural neurosteroid enhancement to ameliorate related comorbidities.





